Exercise: Sensible heating or cooling¶
Pure water temperature¶
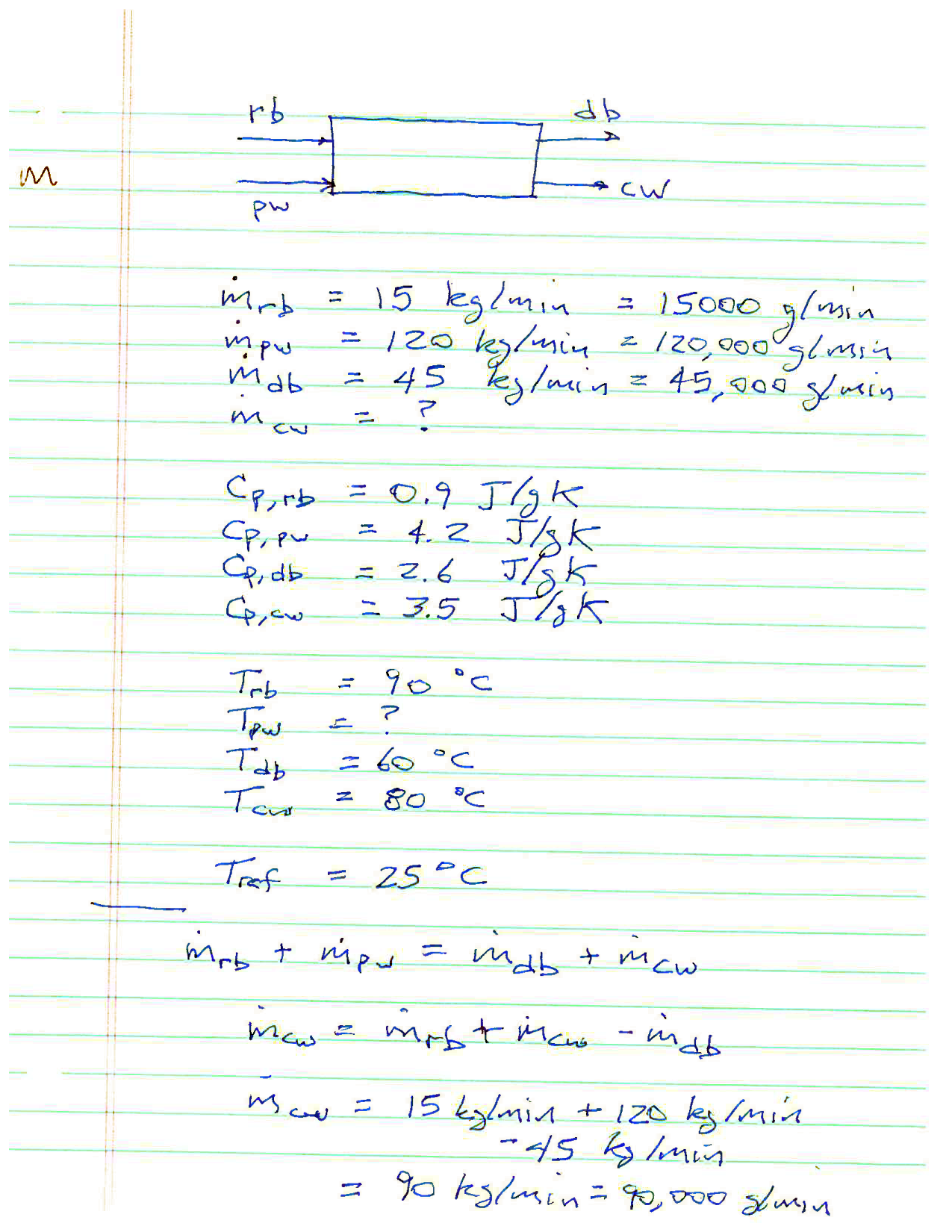

Does this answer seem odd? In particular, how can we ‘combine’ a stream at \(\SI{90}{\degree C}\) and one at \(\SI{65.8}{\degree C}\) and end up with a stream at \(\SI{60}{\degree C}\) and another at \(\SI{80}{\degree C}\)?
For now, instead of thinking about the temperatures, think about the process
of energy transfer in this adiabatic system.
What are the energy rates of the streams?
Essentially to balance these rates (in = out), internal energy must be
transferred between the streams in accord with the specified heat capacities,
flow rates, and temperatures. One stream may have to ‘give up’ internal
energy to satisfy the constraints of the other stream.
In practice, to make this process work, the device would not just be a simple
mixer in which the inlet streams are mixed and somehow separated into the
outlet streams. There would have to be some engineering sophistication with
regard to the mass and heat transfer; however, in theory, it would be possible.
Heat transfer rate¶
Heat must be removed from the system