Process Variables¶
Furious activity is no substitute for understanding.
—H.H. Williams
Terminology¶
Inconsistent notation
One important ‘gotcha’ to keep in mind as you go through your CBE and other technical courses is that the notation is not consistent.
For instance, one book may use the symbol \(\theta\) to represent an angle, while another many use it to represent temperature. A book referring to density may use the symbol \(\rho\), while another may use the Latin letter D.
So, you will need to adapt your notation depending on the context. ☺
Here is a list of a few of the symbols and definitions we will use in this course.
Symbol |
Definition |
Units |
|---|---|---|
\(m\) |
mass |
mass |
\(m_{A}\) |
mass of species \(A\) |
mass |
\(n\) |
moles |
moles |
\(n_{A}\) |
moles of species \(A\) |
moles |
\(V\) |
volume of a process ‘container’ |
volume |
\(MW_{A}\) |
molecular weight of species \(A\) |
mass/mole |
\(\dot m\) |
mass flow rate |
mass/time |
\(\dot n\) |
molar flow rate |
moles/time |
\(\dot V\) |
volumetric flow rate |
volume/time |
\(x_{A}\) |
mass fraction of species \(A\) |
unitless (mass/mass) |
\(y_{A}\) |
mole fraction of species \(A\) |
unitless (moles/moles) |
\(c_{A}\) |
concentration of species \(A\) |
moles/volume |
\(\rho\) |
density |
mass/volume |
\(P\) |
pressure |
force/area |
\(T\) |
temperature |
temperature |
Note that overdots represent rates (quantity per unit time).
More specifically, for the rates, we have the following definitions:
- mass flow rate, \(\dot m\)
the mass of a material that passes a reference plane within a unit time interval
- molar flow rate, \(\dot n\)
the number of moles of a material that passes a reference plane within a unit time interval
- volumetric flow rate, \(\dot V\)
the volume of a material that passes a reference plane within a unit time interval
Useful relationships between some of these variables (KNOW THESE)
These relationships will help convert ‘what you know’ (the givens in a problem) into ‘what you need’ to formulate the equations and solve for the quantities of interest.
Exercise
For the last two equations in the above box, write down the units for each term on the right- and left-hand sides of the equations. Assure that the units are consistent.
Important relationships for mass and mole fractions (KNOW THESE)
It is important to recognize that because they are fractions, the sum of mass (and mole) fractions across the chemical species in a system must be \(1\) .
More formally, given \(N\) chemical species…
Exercise: Calculating process and stream variables
Assuming you know the density of a fluid stream is 2.0 kg/l and the volumetric flowrate is 10 l/hr, write out the equation using the appropriate symbols to find the total mass flowrate and compute the value. Convert units as needed.
You are given the mass fraction of a chemical species in a process stream (0.3) and the total mass flowrate of the stream (100 kg/hr). Write out the equation using the appropriate symbols to find the mass flowrate of the species and compute the value. Convert units as needed.
You know the molar flowrate of a chemical species is 5 gmol/s and the volumetric flowrate is 10 l/s. Write out the equation using the appropriate symbols to find the concentration of the chemical species and compute the value. Convert units as needed.
You are told that the concentration of a chemical species is 2 kgmol/l, the molecular weight of the species is 200 kg/kgmol, and the mass flowate of the species is 800 g/s. Write out the equation using the appropriate symbols to find the total volumetric flowrate of the stream and compute the value. Convert units as needed.
Converting between Mole Fraction and Mass Fraction¶
In general, it is useful to assume some amount of materials for the calculation. We call this the basis of calculation or just basis.
Mole fraction to mass fraction¶
Exercise
As part of a physiology study, you have measured the composition of exhaled gas from a human subject and found the following mole percentages:
\(\ce{O2}\) : \(\SI{18}{mole \, percent}\)
\(\ce{N2}\) : \(\SI{78}{mole\, percent}\)
\(\ce{CO2}\) : \(\SI{4}{mole \, percent}\)
What are the mass fractions of these components?
Mass fraction to mole fraction¶
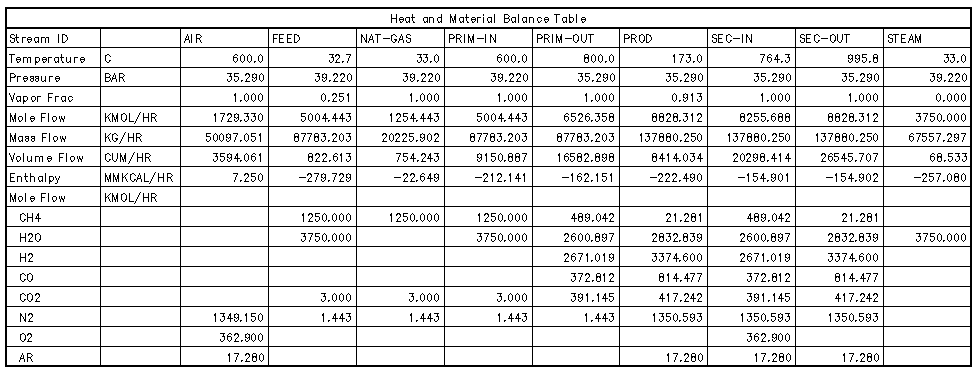
The stream table¶
The stream table lists important variables for all process streams in an organized, tabular format. The rows and columns are customized for the application. Typically, rows would be variables like composition, flow rate, temperature, and pressure; and columns would normally correspond to points before and after pieces of equipment.
A process stream table is also called a process flowsheet.