|
Introduction Typical questions that this section on combustion addresses are:
What are the typical combustion products ? How many moles of each combustion product are produced during a combustion reaction ? Link to: Combustion Products Applet
Air and fuel mix in the piston cylinder to form the reactants of combustion. If we assume all the fuel will be consumed during the reaction, at temperatures less than 1000 K, the chemical reaction can written as:
Equilibrium Combustion Products If the final product temperatures less than 1000 K, the products are CO2, H2O, N2,O2, CO, and H2, with exact concentrations depending on the reaction temperature and fuel/air ratio. At high fuel/air ratios, there is insufficient oxygen to convert all of the carbon in the fuel to carbon dioxide, resulting in the formation of carbon monoxide. At temperatures greater than 1000 K, dissociation reactions during the combustion process will result in additional species, including OH, NO, H and O. In an internal combustion engine, NO is formed during the period of combustion when the reaction temperatures are above 2000 K. The decomposition rate of NO is very slow at temperatures below 2000 K, so the NO concentrations "freeze" at concentrations greater than the equilibrium values at lower temperatures.
Definitions Fuel/Air Ratio
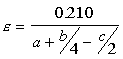 Equivalence Ratio
If the equivalence ratio is equal to one, the combustion is stoichiometric. If it is < 1, the combustion is lean with excess air, and if it is >1, the combustion is rich with incomplete combustion. Fuels Six common fuels are:
C3H8 Propane C2H5OH Ethanol C8H18 Octane
H2
Hydrogen
The solution of for the moles of CO per moles of air, v5, is given by: 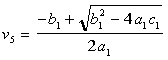
Based on the equivalence ratio the solutions for the molar coefficients of the products are:
The following applet is an extension to the Engine Performance Parameters Applet: Engine Performance Parameters Applet with Fuel Type. This applet compares the effect of fuel type on the performance of the engine while also including the effects of heat transfer. Woschni's correlation for the heat transfer coefficient was used. The following page discusses the method used to determine Qin. |
 |
 |