 |
Introduction
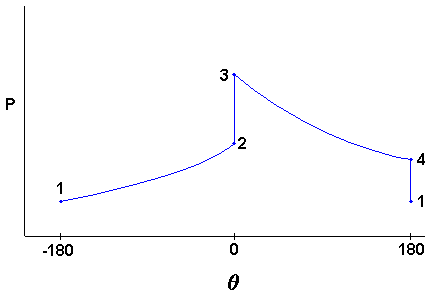
The cycle is named after a German, Nikolaus Otto, who built the first spark ignition engine in 1876. Figure 4 shows schematically how the pressure varies during compression and expansion. From -180 degrees (bottom dead center) to 0 degrees (top dead center) the upward motion of the piston compresses the air/fuel mixture. The heat input for this model is assumed to occur entirely at top dead center. From 0 degrees to +180 degrees the expanding gases move the piston downward and produce work. The heat rejection for this cycle model is assumed to occur entirely at bottom dead center. The air standard Otto cycle is modeled as a closed system with a fixed air/fuel mass, and uses the following process assumptions:
First Law Analysis of the Otto Cycle Using the first law:
Since the process is reversible and adiabatic it is also isentropic, which leads to: 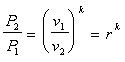 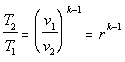
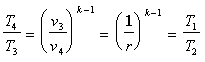
Residual Mass Fraction In an ideal engine all the fuel is consumed in combustion. Realistically
this is not the case there will alwalys be some amount of fuel that will be left
unburned. Due to this residual fuel remaining in the cylinder the intial and exhaust temperatures of the
cycle will not retrun to thier inital value. Assuming the above definitions for the pressure and the
temperature at given points in the cycle, the residual mass fraction can be
given by: Where:
In order to determine the residual mass fraction, the exhaust pressure
and temperature must be known. The exhaust pressure is typically
half of the intake pressure, Pi = 2Pe.
The exhaust temperature can be determined
by assuming that the final mass in the cylinder expands isentropically
to the exhaust pressure. 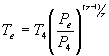 Using the relationships developed for temperatures and pressures from the
first law analysis, the exhaust temperature and residual mass fraction
can be determined by iterating. The Residual
Mass Fraction Applet performs this iteration in order to determine
Te and f, given inital guess for Te and f.
Thermal Efficiency Derivation The overall efficiency for the cycle (indicated thermal 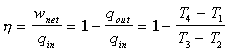 since 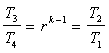 |
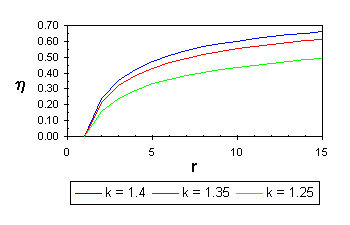 |
The following relationship for the efficiency can be developed and plotted against the compression ratio in Figure 5. A typical value for an air/fuel mixture is k = 1.35. |
|
|
|

|

|