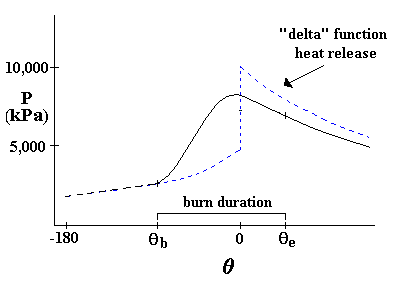

Link to: Heat Release Fraction Applet
Link to: Simple Heat Release Applet
Using the following definitions,![]() heat
release ,
heat
release , ![]() and
and ![]() ,
results in :
,
results in :
The ideal gas equation is PV = mRT, so
and
The first law now becomes
Further reducing the equation:
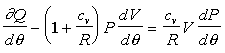
If we know the pressure, P, volume, V, ![]() ,
the heat released gradient,
,
the heat released gradient,![]() , we can compute the change in pressure,
, we can compute the change in pressure, ![]() .
Thus explicitly solving the equation for pressure as a function of crank
angle. Alternatively, we can use experimental data for the pressure, P
and the volume, V, to determine the heat release term by solving for
.
Thus explicitly solving the equation for pressure as a function of crank
angle. Alternatively, we can use experimental data for the pressure, P
and the volume, V, to determine the heat release term by solving for
![]() .
.
First, the volume, V and ![]() , have to be defined. From the slider-crank
model, we have a definition for cylinder volume, V. Both terms
are only dependent on engine geometry.
, have to be defined. From the slider-crank
model, we have a definition for cylinder volume, V. Both terms
are only dependent on engine geometry.
So taking the derivative with respect to the crank angle, ![]() ,
results in :
,
results in :
For heat release term, ![]() ,
the Wiebe function for the burn fraction is used.
,
the Wiebe function for the burn fraction is used.
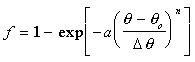
To view the burn fraction, f, as a function of the crank angle, click here: Heat Release Applet.
At the beginning of combustion, f = 0, and at the end the fraction is almost 1.
The heat release, ![]() ,
over the crank angle change,
,
over the crank angle change, ![]() ,
is:
,
is:
Where Qin is the overall heat input.
Taking the derivative of the heat release function, f, with respect
to crank angle, gives the following definition of![]() .
.
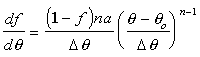
So now with![]() and
and![]() defined, the pressure as a function of the crank angle can be solved.
defined, the pressure as a function of the crank angle can be solved.
The following applet plots the pressure, work and temperature as a function of the crank angle: Simple Heat Release Applet. The effect of heat transfer to the cylinder wall can also be included.
|
|
|

|

|