SPERM CELLS AND SUPERRESOLUTION IMAGING
 SUPERRESOLUTION IMAGING OF CORTICAL ACTIN
SUPERRESOLUTION IMAGING OF CORTICAL ACTIN
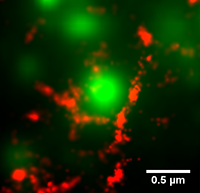
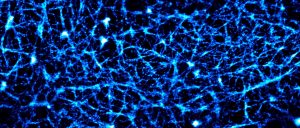
The cortical actin cytoskeleton is a complex, highly mobile network that supports the critical cell function of trafficking, immobilizing, and localizing membrane proteins. Conventional diffraction-limited microscopy cannot resolve the characteristic distances between actin bundles. In our group, we use photoactivated localization microscopy (PALM) to image actin filaments with superresolution. We are investigating the dynamics of the cortical actin and its role in the segregation of membrane proteins into specific microdomains. To date, we have successfully performed simultaneous actin superresolution imaging and single particle tracking of plasma membrane proteins in live human embrionic kidney (HEK).
We use image processing techniques to outline the compartments created by the actin cytoskeleton on the plasma membrane. Using this information, the effect of the compartments on individual membrane proteins can be investigated by combining single particle tracking of membrane proteins with tracks of the individual compartments.
- S. Sadegh, J.L. Higgins, P.C. Mannion, M.M. Tamkun, and D. Krapf, “Plasma Membrane is Compartmentalized by a Self-Similar Cortical Actin Meshwork”, Phys. Rev. X 7, 011031 (2017) pdf
CYTOSKELETON OF SPERM CELLS
The actin cytoskeleton of sperm cells is believed to play fundamental roles in the organization of the flagellum and altering its mechanical properties. However, the architecture of the sperm actin-based cytoskeleton is still poorly understood. Using three-dimensional super-resolution imaging, we have revealed the organization of the actin-based cytoskeleton in the flagellum of mouse sperm. We found that the sperm midpiece (the region of the flagellum closest to the head) and principal piece have specialized actin structures. In the midpiece, polymerized actin forms a double-helix that follows the mitochondrial sheath, a type of filamentous actin structure that had not been previously observed.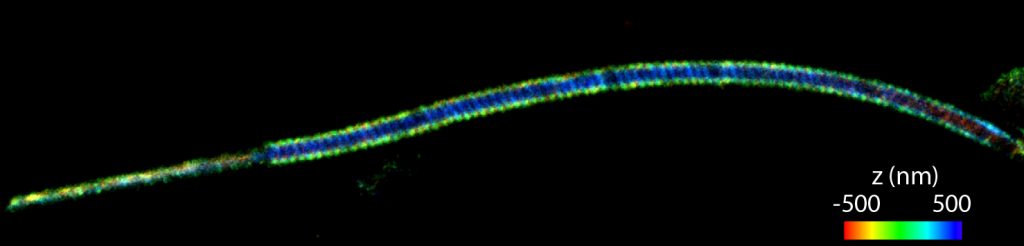
- M.G. Gervasi, X. Xu, B. Carbajal-Gonzalez, M.G. Buffone, P. Visconti, and D. Krapf, “The actin cytoskeleton of the mouse sperm flagellum is organized in a helical structure”, J. Cell Sci. 131 jcs215897 (2018) pdf
- A. Romarowski, A. Velasco Felix, P. Torres Gimenez, M.G. Gervasi, X. Xu, G.M. Luque, G. Contreras Gimenez, C. Sanchez Cardenas, H.V. Ramirez Gomez, D. Krapf, P.E. Visconti, D. Krapf, A.O. Guerrero, A. Darszon, and M.G. Buffone, “Super-resolution imaging of live sperm reveals dynamic changes of the actin cytoskeleton during acrosomal exocytosis” J. Cell Sci. 131, jcs218958 (2018) pdf
MAMMALIAN SPERM CAPACITATION
A key aspect of fertilization involves capacitation, a final sperm maturation step that takes place within the female reproductive tract. In this step, the sperm acquires the capacity to fertilize an egg, but many aspects of capacitation are still largely unknown. The overarching goals of this project are to identify and characterize the mechanisms that regulate sperm capacitation.
- A. Alvau et al., “The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm”, Development 143, 2325 (2016) pdf
- C. Stival, et al., “Disruption of protein kinase A localization induces acrosomal exocytosis in capacitated mouse sperm”, J. Biol. Chem. 293, 9435 (2018) pdf, SI
- G.M. Luque et al., “Cdc42 localized in the CatSper signaling complex regulates cAMP-dependent pathways in mouse sperm”, FASEB J. 35, e21723 (2021) preprint

Krapf Lab © 2018